Grade 7 - Heat and Temperature
Section 1: Using Energy from Heat
Since earlier civilizations, people have used heat (thermal energy) to cook food and to help them stay warm. The same, if uncontrolled, can cause negative effects including burning food or even buildings and homes.
Humans have been developing efficient ways of heating homes and cooking food. Technology has improved and the pan of water that boiled dry over the cookfire has been replaced by a kettle placed on a gas or electric stove that whistles when the water boils. Many other technologies also use heat to make lives efficient or more comfortable such as hair dryers, clothes dryers, ovens etc.
Section 2: Measuring Temperature
Temperature is a measure of how warm or cool an item or a surface is. A simple way to estimate temperature is just to touch the item/surface. The human skin has nerve endings that can sense different temperatures, so people can learn to recognize the feeling of particular temperatures by touching. Hospital and health care workers can detect dangerous body temperatures by touching a patient’s forehead with the back of a hand. In this case, the workers just need to detect hotter or cooler than normal temperatures without necessarily obtaining an accurate measure of the temperature. Some materials change color when they are hot and this can give an indication of how hot those materials are. On the other hand, some materials will change from solid to liquid or gas depending on how hot (or cold) they are. Welders and glass blowers can estimate when a flame is hot enough to soften metal or glass. Astronomers judge the temperature of stars by the colour of the light they emit. These methods of measuring temperature are not necessarily accurate but can help estimate the temperature of a surface or an object.
Thermometers
Thermometers are devices for measuring temperature, they may use mechanical or electronic technologies underlying the detection method. As people discover the effects of temperature, there is need to measure temperatures more accurately. Modern thermometers have gradations or evenly spaced lines that allow you to measure temperatures more accurately.
There are several scales for measuring temperature, however three are most popularly used. These include the Celsius scale, Fahrenheit scale and the Kelvin scale. The temperature scale commonly used in Canada and many other countries is called the Celsius scale in honour of Anders Celsius (1701–1744). He used the 'degree' as the unit of temperature and based his standards for comparison on the properties of water. The temperature at which ice melts at sea level was assigned 0°C (freezing temperature) and the temperature at which liquid water boils into steam at sea level at sea level was assigned 100°C (boiling temperature). Then he separated the region between these temperatures into 100 evenly spaced units or degrees. To be accurate, this type of calibration must be done at sea level using very pure water.
Impurities in water affect its boiling and freezing points. For example, salt water only saltwater that’s as saturated as it can possibly get (i.e. there’s no way to dissolve any more salt in it no matter how hard you tried), the freezing point is -21.1°C.
Pressure also affects the boiling point and freezing point of water. Extremely high pressures cause ice to melt even at temperatures below 0°C. In Alberta, for example, the high altitude means that the weight of the air above you is smaller than it would be at sea level. As a result, water in Alberta boils at several degrees less than 100°C. At the top of Mount Everest, water would boil at only 69°C.
To study the behaviour of gases at different temperatures, there was need to develop a temperature scale that started at the coldest possible temperature, also called “absolute zero.” The temperature scale that defined the absolute zero is called the Kelvin scale, in honour of William Thomson (1824–1907), who was given the title Lord Kelvin. Although no one has ever been able to cool anything down to absolute zero, scientists predict that the temperature is –273°C.
The units of temperature on the Kelvin scale are called Kelvins. For example, the freezing temperature of water at sea level is 273K.
Thermometers have been developed to be useful in almost every purpose, from measuring the extreme cold of outer space to estimating the temperatures of stars. Every thermometer contains a sensor — a material which is affected by changes in temperature. The sensor produces a signal — such as an electrical current, indicative of the temperature. The signal affects a responder — a pointer, light, or other mechanism that interprets the signal and displays it.
Types of Thermometers
1. The Thermocouple
In a thermocouple, wires made of two different metals are twisted together. When the twisted wire is heated, a small electrical current is generated. The amount of current depends on the temperature of the wires. The electrical current from the thermocouple can be used to turn a switch or a valve on or off if the temperature changes. Thermocouples can measure extremely high temperatures that cannot be measured by other thermometers.
2. Bimetallic Strip
As the name suggests, a bimetallic strip is made of two different metals joined firmly together. As the strip is heated, one metal expands more than the other. The strip is forced to coil more tightly. When the strip cools, the process is reversed. The same metal that expanded rapidly now contracts rapidly and the strip uncoils again.
Movements of the bimetallic strip can operate a type of electrical switch, (thermostat) which can be used to control furnaces, air conditioners, refrigerators, or other devices.
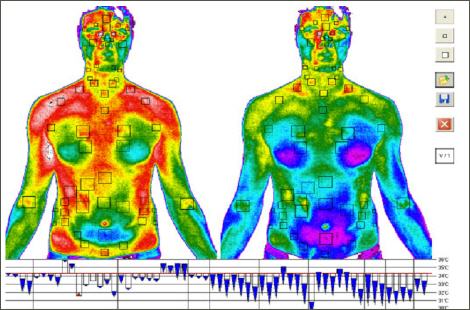
3. Infrared Thermography
Infrared (IR) thermography is the acquisition and analysis of thermal information from non-contact thermal imaging devices. IR thermography detects emitted radiation in the infrared range of the electromagnetic spectrum. This corresponds to wavelengths longer than the visible light portion of the spectrum. Infrared radiation can be photographed with special films or detected by electronic sensors that display images on television screens, or interpreted and displayed as a number in °C or °F. IR images use colour or brightness of the image to show the temperature of the object.
Section 3: The Particle Model
The Particle Model of Matter is a scientific description of the tiny particles that make up all things. The key elements in this model include:
- All substances are made of tiny particles
- Particles are always in motion
- The particles have spaces between them
Temperature and the Particle Model
The motion of a substance's particles are affected by the temperature. When heat is added, the particles move faster. When heat is lost, the particles move slower. Therefore, the motion of the particles increases when the temperature increases. The motion of the particles decreases when the temperature decreases. Temperature indicates the average energy (speed) of the particles in motion in a substance.
Energy
Energy is the ability to perform work, or to change an object. Work is the measurement of the energy used to peform a task. Work is a product of the force used to perform the task and the distance the force was applied. Therefor the units of measurement of work and energy are Newton.meters (N.m). If you lift a box that weighs 10 newtons onto a shelf that is 2 meters high, you are performing 20 N∙m of work (10*2). N.m are also known as joules (J). In describing how you lifted and moved the box, you could also say that you used 20 J of energy.
Thermal Energy
Thermal energy is caused by the motion of particles in matter. Heat is the flow of thermal energy from one object to another due to the difference in their thermal energies. Thermal energy moves from an object of higher thermal energy to an object of lower thermal energy.
Temperature is a measurement of the average kinetic energy of particles in an object. objects with a higher temperature have particles that are moving faster, and vice versa. When two objects of different temperatures come into contact, thermal energy is transferred from the warmer object to the cooler object so the warmer object cools down while the cool object warms up to an equilibrium.
When two objects rub against each other, they become warm, because the kinetic energy changes into thermal energy.
Temperature is measured using a thermometer.
Please note that temperature and thermal energy are not the same thing. Thermal energy is the total amount of energy in a material due to the motion of the particles in that material. Temperature is a measure of the average kinetic energy of the material.
How does energy travel?
There are three main mechanisms in which thermal energy can be transferred from one object to another. These include:
- Conduction: Conduction occurs when thermal energy is passed through a material while the material itself remains in place. The two objects must be in contact.
- Convection: This is the flow of energy through a liquid or gas caused by the hot parts rising and the cool parts of the liquid or gas sinking. hot air and liquid becomes less dense and rises while cool air/liquid is more dense so it sinks. as this occurs, the thermal energy is transfered from the warmer particles to the cooler particles.
- Radiation: this is the transfer of thermal energy through electromagnetic rays. These include X-rays, visible light and radio waves.
The ability of a material to transfer thermal energy is called thermal conductivity. If a material conducts energy easily, it is described as a good thermal conductor. If a material conducts energy poorly, it is a good thermal insulator. Most metals are good thermal conductors, and most nonmetals are good thermal insulators. Thermal conductivity is usually highest in solids and lowest in gases. Solids are better conductors than liquids. Liquids are better conductors than gases. This is because, the closer particles are together, the more particles can bump into each other and transfer energy.
Energy is not a substance. It cannot be seen, weighed or take up space. Energy is a condition or quality that a substance has. Energy is a property or quality of an object or substance that gives it the ability to move, do work or cause change.
Section 4: Thermal Expansion and Contraction
Materials expand or contract when subjected to changes in temperature. Most materials expand when they are heated, and contract when they are cooled. When free to deform, concrete will expand or contract due to fluctuations in temperature. The size of the concrete structure whether it is a bridge, a highway, or a building does not make it immune to the effects of temperature. The expansion and contraction with changes in temperature occur regardless of the structure's cross-sectional area.
Contraction in Solids
Concrete expands slightly as temperature rises and contracts as temperature falls. Temperature changes may be caused by environmental conditions or by cement hydration (the exothermic chemical process in which the cement reacts with the water in a mixture of concrete to create the calcium silicate hydrate binder and other compounds). An average value for the coefficient of thermal expansion of concrete is about 10 millionths per degree Celsius (10x10-6/C), although values ranging from 7 to 12 millionths per degree Celsius have been observed. This amounts to a length change of 1.7 centimeters for every 30.5 meters of concrete subjected to a rise or fall of 38 degrees Celsius.
Thermal expansion and contraction of concrete varies primarily with aggregate type (shale, limestone, siliceous gravel, granite), cementitious material content, water cement ratio, temperature range, concrete age, and ambient relative humidity. Of these factors, aggregate type has the greatest influence on the expansion and contraction of concrete.
Severe problems develop in massive structures where heat cannot be dissipated. Thermal contraction on the concrete’s surface without a corresponding change in its interior temperature will cause a thermal differential and potentially lead to cracking. Temperature changes that result in shortening will crack concrete members that are held in place or restrained by another part of the structure, internal reinforcement or by the ground. For example, a long restrained concrete section is allowed to drop in temperature. As the temperature drops, the concrete tends to shorten, but cannot as it is restrained along its base length. This causes the concrete to be stressed, and eventually crack.
Joints are the most effective way to control cracking. If a sizable section of concrete is not provided with properly spaced joints to accommodate temperature movement, the concrete will crack in a regular pattern related to the temperature and restraint directory. Control joints are grooved, formed, or sawed into sidewalks, driveways, pavements, floors, and walls so that cracking will occur in these joints rather than in a random manner. Contraction joints provide for movement in the plane of a slab or wall, and induce cracking caused by thermal shrinkage at preselected locations. One of the most economical methods for making a contraction joint is by simply sawing a continuous cut in the top of the slab with a masonry saw.
Expansion and Contraction in Gases
Because most common gases are colourless, they are difficult to observe. As well, gases have no fixed shape or size. (Remember that they always take the shape and size of their container.) If you put gases in a flexible container such as a balloon, however, you can see that they expand and contract much more than solids when the temperature changes. Warming a sample of helium from 0°C to 100°C, for example, increases its volume by about one third. Unlike the particles in solids, the particles in gases are far apart and moving fast and freely.
Expansion and Contraction in Liquids
As the thermometer liquid moves up the glass tubing (the bore), it takes up more space. In other words, the liquid expands as it warms. As the thermometer cools, the liquid contracts, so it moves back down the tubing. The liquid must be contracting as it cools. Do all liquids expand and contract in this way? Do some liquids change volume more than others as they warm and cool? Follow the next activity carefully to find out.
Section 5: The Particle Model and Changes of State
Temperature is a measure of how hot or cold something is. It is a measure of the average amount of kinetic energy of the atoms and molecules in a material. When you hang clothes to dry, the heat energy from the sun heats up the water in the clothes causing it to evaporate (change from liquid to gas (vapor). A change from one state to another involved either loss or gain of energy by the substance. When matter gains energy, its particles move faster resulting in more collisions between particles. The body uses up heat energy from the surface of the skin to evaporate sweat, this loss of heat energy is intended to make you feel cooler. Sweating, therefore, is a biological process which allows you to cool down when its hot outside.
The state of matter can change in other ways. Sublimation occurs when a substance changes directly from a solid to a gas without going through a liquid state. Ice cubes left in your freezer for a long period of time will become smaller. Eventually, the ice cubes will 'disappear'. This is because the water molecules in the ice cubes change directly from the solid state (ice) to the gaseous state (water vapor).
If you heat a solid substance, the particles move faster and if there is enough energy, the solid will melt into a liquid. Melting is the change of state from solid to liquid. If you heat the liquid farther, the particles move even farther apart and the liquid changes to a gas. This process is called Boiling. The temperature at which a liquid becomes a gas is its boiling point. Evaporation is that process where a liquid can change to a gas without boiling. Water on ponds, lakes, rivers and oceans is constantly undergoing evaporation.
Cooling occurs when we take away heat energy from a substance. This makes the particles move closer together. If we cool a gas into a liquid, we call that Condensation. Or we can say the gas condenses to a liquid. If we cool a liquid into a solid (like if we put water into the freezer), we call that Freezing, or we can say the liquid freezes into a solid. Water freezes into ice. Sublimation occurs when a gas changes directly to a solid or a solid changes directly to a gas.
Matter can be found in three common states namely: solids, liquids and gases.
Solids: Solids have a shape and take up a definite amount of space. In solids, the particles of matter are packed tightly and mostly in a regular pattern. The pencil, pen, book, desk, blocks, wood, ice ... are all solids.
Liquids: Liquids do not have a definite shape, they take the shape of the container. Juice is a liquid, if you pour it into a glass, it will spread out and take up the shape of the glass. In liquids, the particles that make up matter are farther apart and can move more freely than in solids. Water, juice, milk, and oil are examples of liquids.

Gases: If you pour juice into a glass, it goes to the bottom of the glass makes the glass half full. Gases do not have a definite shape. In addition, if you put a gas into a container, it spreads out throughout the container. In gases, the particles spread out so as to fill the space in the container. If the space is small, the particles will be tight together, if the space is big, the particles will be spread out farther apart. Air is mostly made out of gases.
The particles present in a drop of a liquid are moving at many different speeds. On the surface of the drop, some of the faster-moving particles are able to escape into the air while slower-moving particles stay in the liquid state. Slower motion means lower temperature. As high-energy particles evaporate, the remaining liquid cools. The cool liquid then cools the surface on which it is resting. This phenomenon is called evaporative cooling.
The Anomalous Behavior of Water
At moderate temperatures, liquids expand when heated and contract when cooled. Water, on the other hand, behaves strangely. It compresses when heated between 0 and 4 degrees Celsius. As a certain amount of water is cooled from room temperature to 4 degrees Celsius, its volume decreases. When the temperature drops below 4 degrees Celsius, the volume expands and the density decreases. This indicates that the water's maximum density is at 4 degrees Celsius. Abnormal expansion of water is the term for this type of water phenomena.
Transfer of Energy
There are three main mechanisms in which thermal energy can be transferred from one object to another. These include:
- Conduction: Thermal conduction is the process of transferring thermal energy through direct collisions between particles. The two objects must be in contact. Most metals, especially gold and copper, are excellent heat conductors. A hot stove burner touching one part of a copper saucepan, for example, soon heats the entire pan. Other solids, such as glass and wood, are much less efficient at transferring thermal energy by conduction. Poor conductors are called heat insulators. When insulators are wrapped around an object, they slow down the transfer of thermal energy to or from the surroundings.
- Convection: This is the flow of energy through a liquid or gas (fluids) caused by the hot parts rising and the cool parts of the liquid or gas sinking. Hot air and liquid becomes less dense and rises while cool air/liquid is more dense so it sinks. as this occurs, the thermal energy is transfered from the warmer particles to the cooler particles. The moving fluid is called a convection current. materials expand as they warm up. Their particles move farther apart. Each section of the warmed material is left with fewer particles than when it was cold, so each section is a bit lighter than it used to be. In other words, the warmed material becomes less dense. Colder, denser fluid sinks down and pushes nearby warmer fluid upward. Then this cold fluid, too, is warmed and pushed upward.
- Radiation: Radiation is the transfer of energy without any movement of matter. Energy that is transferred in this way is called radiant energy or electromagnetic radiation. There are many different forms of EMR, including radio waves, microwaves, visible light, and X-rays. If the energy source is a warm object, such as the Sun, some of its thermal energy is transferred as a type of EMR called infrared radiation (IR) or 'heat radiation.'
Energy Transfer Systems
All energy transfer systems have 5 things in common:
- Energy Source Some part of the system acts as an energy source, supplying energy to the rest of the system.
- Waste Heat Almost all energy systems transfer at least a little thermal energy into the surroundings.
- A control systems: For example a thermostat controls energy transfer by turning the furnace on and off.
- Energy Transformations: Energy changes form as it is transferred from place to place.
- Direction of Energy: Transfer Energy is always transferred away from concentrated sources.